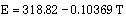 (1)
(1)
Molybdenum and copper alloys are considered candidate materials for the heat-sink system of the divertor of ITER. The design which includes graphite tiles on molybdenum substrate has been prefered for the physics stage of the divertor, ref [1]. The main advantage of such a solution is the similarity of the coefficients of thermal expansion for molybdenum and graphite, which permits significant reduction in the levels of thermal stresses. A high level of strength, high resistance to radiation swelling, and good thermal conductivity are among the advantages of using molybdenum alloys.
The principal disadvantage of molybdenum-base alloys is a tendency to low-temperature embrittlement. Therefore it was necessary to find a solution to reduce the ductile to brittle transition temperature (DBTT) and to provide a minimal shift of the DBTT following low-temperature neutron irradiation.
It has been known for a long time that Mo-Re alloys provide satisfactory properties arising from the rhenium addition to molybdenum. Mo-Re alloys at 3-7% Re concentrations exhibit a significant increase in ductility in low-temperature tests, ref [2]. Due to the refining effects of Re on the microstructure the strength of welded joints of Mo-Re alloys is improved significantly, mainly because of the grain size reduction in the heat affected zone at ~5 % Re, ref [3].
Fundamental studies in the 1950's on the mechanisms controlling the use of pure molybdenum revealed that cold working was effective in improving fabricability and that small additions of reactive elements increased the elevated temperature capability by raising the recrystallization temperature. A Mo-0.5Ti-0.08Zr (TZM) alloy is representative of this class of materials.
Radiation effects
Research into the effects of neutron irradiation on the mechanical properties of molybdenum alloys revealed a wide variation depending on the initial condition of the starting material and the material composition. For TZM the data ranges from 0.7 to 4 dpa and irradiation temperatures of 370-550 C, ref [4]. Analysis of this data led to the conclusion that at ~370 C the strength of annealed TZM after irradiation approached the strength level of the stress relieved material. Irradiation at higher temperatures (550 C) resulted in ductilities below 5%. It was also anticipated that there would be a corresponding increase in DBTT to well above room temperature.
GENERAL PROPERTIES - TZM [8]
Physical properties
Boiling point : 4612 C
Density @ 20 C : 10.22 g/cm3
Melting Point : 2500 - 2600 C
Electrical Properties
Electrical Resistivity @ 20 C : 5.3 - 5.5 u [[Omega]] cm
Thermal Properties
Linear Expansion Coefficient
@ 20 - 100 C : 5.3*10-6 K-1
Thermal Conductivity @ RTP : 126 W/m-K
Maximum Use Temperature in Air : 400 C
Mechanical Properties
Elongation : < 20 %
Modulus of Elasticity : 320 GPa
Tensile Strength : 560 - 1150 MPa
DATA AND CORRELATIONS
MOLYBDENUM ALLOY TZM
The thermal and structural properties of TZM as a function of temperature are presented in Table 1, refs [4, 6, 7]. Polynomial correlations of the thermal and structural properties as functions of temperature, using the data of Table 1, are as follows:
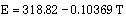 (1)
(1)
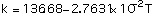 (2)
(2)
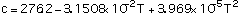 (3)
(3)
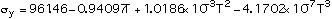 (4)
(4)
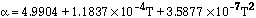 (5)
(5)
Equations (1), (2), (3) and (5) are valid between 300-2700 K, and Eq (4) between 300-1800 K.
PURE MOLYBDENUM
Data for pure molybdenum are shown in Table 2, taken from refs [8, 9, 10]. The following expressions are polynomial curve fits to the data for the thermal and structural properties of pure molybdeum, given as functions of temperature:
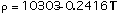 (1)
(1)
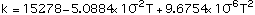 (2)
(2)
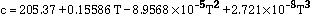 (3)
(3)
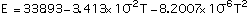 (4)
(4)
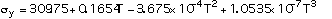 (5)
(5)
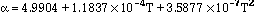 (6)
(6)
Equations (1), (4) and (6) are valid in the temperature range 300-2900 K, Eqs (2) and (3) in the range 300-2600 K and Eq (5) in the range 300-2100 K.
TABLE 1 Thermal and structural properties of TZM
T rho E nu k c sigmay alpha
K kg/m3 GPa W/m-K J/kg-K MPa (10-6)m/m-K
300 10200.0 284.330 0.33 127.509 272 759.589 5.080
400 274.930 125.457 271 721.360 5.120
600 255.860 120.530 272 673.500 5.230
700 246.190 118.440 274 658.860 5.290
800 236.440 115.510 276 647.080 5.370
900 226.590 113.000 280 635.650 5.450
1000 216.660 110.070 285 622.070 5.530
1200 196.530 103.790 295 578.480 5.730
1500 165.660 94.580 318 434.410 6.070
1600 155.190 91.230 326 355.410 6.200
1900 123.260 82.860 362 6.630
2000 112.440 80.350 372 6.790
2100 101.530 77.840 387 6.960
2500 57.000 69.050 446 7.690
2600 45.650 67.380 462 7.890
TABLE 2 Thermal and structural properties of pure molybdenum
T rho E nu k c sigmay sigmau alpha
K kg/m3 GPa W/m-K J/kg-K MPa MPa (10-6)m/m-K
300 10240.0 330.0 0.356 138.00 251.0 330.00 659.91 5.08
400 10204.0 323.47 134.00 261.0 315.35 630.67 5.12
600 10156.0 311.84 126.00 275.0 295.00 590.44 5.23
700 10134.0 306.61 122.50 279.09 282.59 565.17 5.29
800 10108.0 301.68 118.00 285.0 265.79 531.60 5.37
900 10086.0 297.00 114.82 290.73 245.00 488.91 5.45
1000 10060.0 292.45 112.00 295.0 160.66 438.03 5.53
1200 10012.0 283.64 105.00 308.0 160.00 321.44 5.73
1500 9940.0 270.00 98.00 330.0 80.00 159.17 6.07
1600 9916.0 265.14 96.13 337.82 59.973 120.28 6.20
1900 9843.0 248.43 91.03 367.46 28.00 56.51 6.63
2000 9820.0 242.0 90.00 380.0 20.40 41.51 6.79
2100 9796.0 234.95 88.59 392.53 8.50 17.05 6.96
2500 9700.0 200.00 86.00 459.0 7.69
2600 9676.0 189.22 85.89 480.02 7.89
k (W/m-K) c (kJ/kg-K)
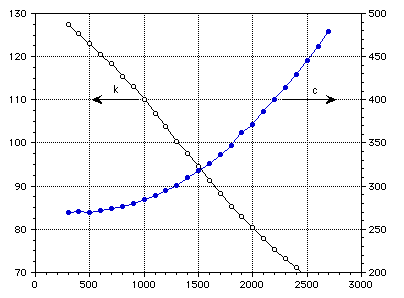
Temperature (K)
Figure 1 : Thermal conductivity and specific heat of TZM.
E (GPa) [[alpha]] (10-6 m/m-K)
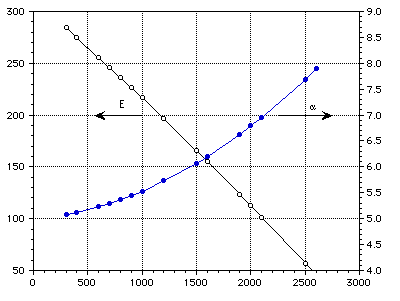
Temperature (K)
Figure 2 : Elastic modulus and coefficient of thermal expansion of TZM.
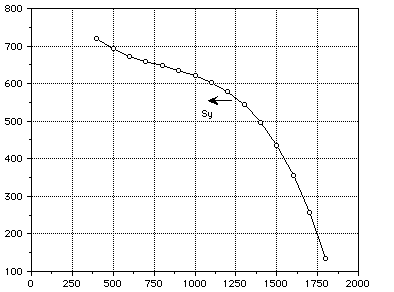
Temperature (K)
Figure 3 : Yield Stress of TZM.
k (W/m-K) c (J/kg-K)
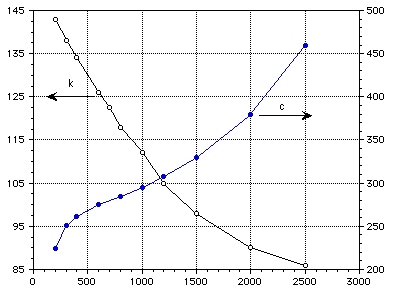
Temperature (K)
Figure 4 : Thermal conductivity and specific heat of pure molybdeum.
E (GPa) [[alpha]] (10-6 m/m-K)
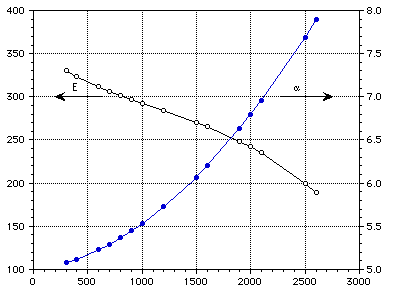
Temperature (K)
Figure 5 : Elastic modulus and coefficient of thermal expansion of pure molybdeum.
[[rho]] (kg/m3) [[sigma]]y (MPa)
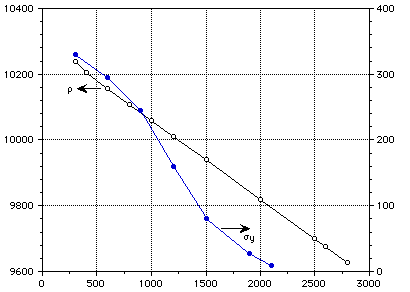
Temperature (K)
Figure 6 : Density and yield stress of pure molybdeum.
Ivica Smid, Masato Akiba, Masanori Araki, Satoshi Suzuki and Kazuyoshi Satoh. Material and Design Considerations for the Carbon Armored ITER Diverter, July 1993.