 (1)
(1)
Lithium oxide (Li2O) with its high tritium breeding potential is an attractive candidate for the solid breeder material of a D-T fusion power plant, because of its high lithium atom density (compared to other lithium ceramics or metallic lithium) and its relatively high thermal conductivity.
Li2O will be exposed to high temperatures under neutron irradiation during the operation of fusion blankets. Under these circumstances, a huge number of irradiation defects will be produced in Li2O, such as helium-induced swelling, relatively high thermal expansion, grain growth, LiOH(T) formation and precipitation at low temperatures, and LiOH(T) mass transport at high temperatures. Furthermore, Li2O will be subjected to stresses arising from differences of thermal expansion between Li2O and structural materials. These characteristics of Li2O lead to challenging engineering problems both in fabrication and blanket design.
GENERAL PROPERTIES - LITHIUM OXIDE [1]
Pure lithium oxide has a face-centered cubic structure . It melts at 1432 C and exhibits no phase changes from room temperature to the melting point.
Lithium oxide readily reacts with water vapour to form hydroxide, and with carbon dioxide to form carbonate; consequently, it must be stored and handled in a clean and dry atmosphere.
Physical properties
Density @ 25 C : 2.013 g/cm3
Melting Point : 1432 +/- 6 C
Thermal properties
Specific Heat @ 25 C : 2049 J/kg-K
Thermal Conductivity @ 100 oC : 11.29 W/m-K
Mechanical properties
There is very little or no data in the literature, on the mechanical properties of Lithium Oxide. Some results of the measurements made at NRL-S , ref [2], are as follows:
TD* % Elastic modulus GPa Compressive strength MPa Poisson ratio
[[nu]]
Static Dynamic
81.4 76 72 +/- 5 132 0.18
91.5 --- 114 +/- 10 139 0.13
*TD=Theoretical density
DATA AND CORRELATIONS
The effect of porosity on thermal and physical properties of ceramics is very pronounced and reflected in the available data. Thermal conductivity k (W/m-K) is affected by irradiation, porosity and morphology and has been expressed as follows, refs [3, 4]:
 (1)
(1)
with p the porosity. Another expression from ref [5] is as follows:
 (2)
(2)
with D = Bulk density / Theoretical density. All temperatures in Eqs 1-2 are in degrees K.
Table 1 presents the thermal and structural properties for lithium oxide as a function of temperature, refs [3, 4, 6, 7]. Polynomial correlations of the thermal and structural properties as functions of temperature in degrees K, are as follows:
 (2)
(2)
 (4)
(4)
 (5)
(5)
Equations (1) to (4) are valid in the temperature range 300-1100 K.
Figures 1-2 show the variation of properties with temperature and Figs 3-4 show the effect of porosity on the thermal conductivity. Fig 3 shows a comparison between experimental data and the semi-empirical expressions of Eq 1, from refs [2, 3, 5].
Table 1 Thermal and structural properties of lithium oxide
T K [[rho]] E GPa [[nu] k W/m-K c J/kg-K [[sigma]]y [[alpha]]
kg/m3 ] MPa (10-6)
m/m-K
300 2010.0 140.822 0.2 14.526 1686.344 25.910
350 139.553 12.823 1950.142 26.770
400 138.284 11.478 2127.200 27.630
450 137.015 10.388 2253.825 28.490
500 135.746 9.487 2349.140 29.350
550 134.477 8.730 2423.993 30.210
600 133.208 8.085 2484.911 31.070
650 131.939 7.528 2536.012 31.930
700 130.670 7.044 2579.998 32.790
750 129.401 6.618 2618.701 33.650
800 128.132 6.240 2653.400 34.510
850 126.863 5.903 2685.009 35.370
900 125.594 5.601 2714.194 36.230
950 124.325 5.328 2741.453 37.090
1000 123.056 5.081 2767.160 37.950
1050 121.787 4.855 2791.604 38.810
1100 120.518 4.649 2815.011 39.670
1150 119.249 4.459 2837.555 40.530
1200 117.980 4.285 2859.378 41.390
k (W/m-K) c (J/kg-K)
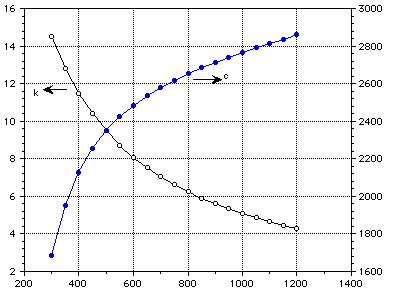
Temperature (K)
Figure 1: Thermal conductivity and specific heat of lithium oxide.
E (GPa) [[alpha]] (10-6 m/m-K)
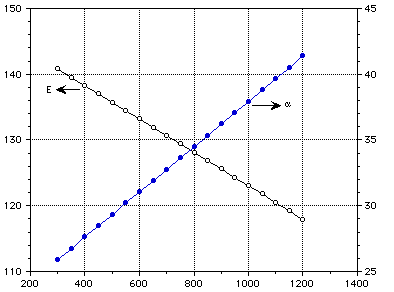
Temperature (K)
Figure 2: Elastic modulus and thermal expansion coefficient of lithium oxide as a function of temperature.
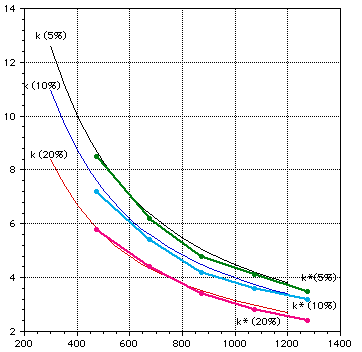
Temperature (K)
Figure 3: Comparison of theoretical and measured (thick lines) thermal conductivity of lithium oxide.
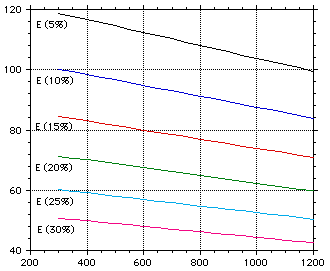
Temperature (K)
Figure 4: The elastic modulus of lithium oxide with the effect of porosity.