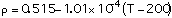 (2)
(2)
The use of lithium or lithium-containing materials as tritium generating or coolant materials has been central in all D-T fusion plant design studies.
Lithium acts as a tritium generating material, when neutrons react with 6Li (n, [[alpha]]) T and 7Li (n, n'[[alpha]]) T. In addition, liquid lithium and some molten lithium salts which have favorable heat transfer characteristics are proposed as potential coolants.
The compatibility of liquid lithium with various metals and ceramics at elevated temperatures depends to a significant extent on the concentrations of various impurities in the lithium and in the metal or ceramic. The effect of a given impurity concentration on the corrosion rate depends upon the thermodynamic and phase relationships for the impurity-lithium, impurity-metal and impurity-ceramic system, with the most important impurities being hydrogen, deuterium and tritium.
The solubility of refractory metals and their alloys with lithium is an important factor in deciding which metals are suited, if lithium was to be as a coolant in the fusion reactor.
GENERAL PROPERTIES - LITHIUM [1]
Physical properties
Boiling point : 1342 C
Density @ 20 C : 0.534 g/cm3
Melting Point : 180.5 C
Electrical properties
Electrical Resistivity @ 20 C : 9.29 u[[Omega]] cm
Cold Junction @ 0 C,
Hot Junction @ 100 C : +1.82 mV
Temperature Coefficient @ 0 - 100 C : 0.00435/K
Thermal properties
Latent heat of Evaporation : 19600 J/g
Latent heat of fusion : 422 J/g
Linear Expansion Coefficient
@ 0 -100 C : 56x10-6 /K
Specific heat @ 25 C : 3560 J/kg-K
Thermal Conductivity, @ 0 - 100 C 84.8 W/m-K
Mechanical properties
Material Condition Polycrystalline
Bulk modulus (GPa) 11.1
Hardness-Vickers < 5
Poisson's Ratio 0.36
E (GPa) 4.91
COMPATIBILITY WITH REFRACTORIES AND
CERAMIC INSULATORS
The compatibility of lithium with various materials is an important issue for the design of a fusion power plant. The solubilities of selected refractory metals in lithium are summarized in Table 1 and the compatibility with various ceramic insulators in Table 2.
In general the metals which are most resistant to attack by lithium at temperatures close to 1000 C are those of groups IVB, VB, VIB, and VIIB of the periodic table. Those which have received the most attention are Ti, Zr, Hf, V, Nb, Ta, Mo, W, Re and their alloys.
It has been demonstrated that lithium is extremely aggressive towards ceramics, mostly oxides, although double metal oxides are generally more stable than the single oxides from which they are prepared. Certain ceramics, such as high purity BeO, Y3Al5O12, ThO2, MgO.Al2O3, AlN, and Y2O3, have shown promise in resisting corrosion by lithium [2].
DATA AND CORRELATIONS
Thermophysical data has been collected on pure liquid lithium with respect to temperature, from ref [3-8] shown in Table 3 and Figs 1-2. Empirical equations for some physical and thermal properties of Lithium as a function of temperature are as follows:
Density [[rho]] in g/cm3:
rho = 0.5082 - 1.0336x10-4 T* - 4.8279x10-10 T*2 - 5.2853x10-12 T*3 (1)
with
T* = (T - 271.7)
and
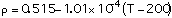 (2)
(2)
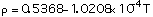 (3)
(3)
with T in degrees Celcius, Eq (1) is valid for temperatures between 300 - 1000 C, Eq (2) between 200 - 1600 C and Eq (3) between 400 - 1125 C.
Thermal Conductivity k in cal/sec-m-C, refs [4,9,10]:
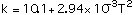 (4)
(4)
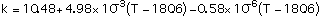 (5)
(5)
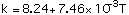 (6)
(6)
with T in degrees Celcius, Eq (4) valid for temperatures between 250 - 950 C, Eq (5) between 300 - 1100 C and Eq (6) between 320 - 830 C.
Viscosity in centipoise:
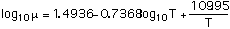 (7)
(7)
with T in degrees Kelvin.
Enthalpy in cal/g:
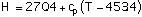 (8)
(8)
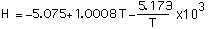 (9)
(9)
with T in degrees Kelvin in Eq (8) and range 190-650 C, degrees Celsius in Eq (9) and range 500-1300 C and the specific heat at constant pressure in the range 600-1000 C constant with cp=0.995 cal/g-K.
Table 1 Solubilities of selected refractory metals in lithium
Solute Solubility ppm Temperature C
Ti 345* 730
Ti 3700* 1020
Mo < 15* 550
Mo < 25* 860
V 150* 725
V 65* 1010
W 1050* 715
W ~ 3^ 1200 - 1600
*
(oxygen impurity level of Li: 2400 ppm), [11].^ (impurity levels of Li: 44 ppm C, 13 ppm N, 33 ppm O), [12].
Table 2
Sample Temp (C) Time (hr) Results
BeO 1093 500 Significant attack, Li penetration
MgO 1093 100 Disintegrated
Y2O3 1093 1000 Slight attack
ThO2 1093 1000 Slight attack
ThO2 -5% Y2O3 1093 1000 Very minor attack
AlN 1093 1000 Severe cracking
BN 1093 100 Disintegrated
TiC 1093 2000 Some cracking
MgAl2O4 1093 100 Disintregated
TABLE 3 Thermophysical properties of pure liquid lithium
T C [[rho]] [[nu]] k W/m-K C J/kg-K [[eta]] [[alpha]]
kg/m3 mPas (10-6)
m/m-K
250 510.0 0.360 45.364 4.308 283.913
300 505.0 45.979 4.270 333.456
350 500.0 46.595 4.240 383.222
400 495.0 47.210 4.215 433.043
450 490.0 47.826 4.198 482.915
500 485.0 48.441 4.183 532.819
550 480.0 49.057 4.172 582.745
600 475.0 49.672 4.165 632.685
650 470.0 50.288 4.158 682.636
700 464.5 50.903 4.153 732.600
750 459.0 51.519 4.151 782.562
800 454.0 52.134 4.148 832.532
850 449.0 52.749 4.147 882.507
900 444.0 53.365 4.146 932.485
950 439.0 53.980 4.146 982.466
k (W/m-K) c (J/kg-K)
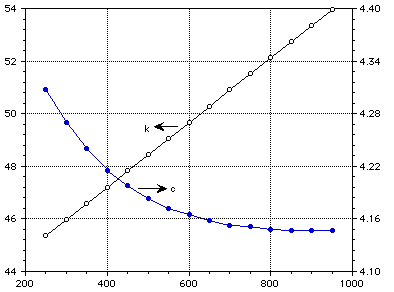
Temperature (C)
Figure 1 : Thermal conductivity and specific heat of pure liquid lithium.
[[rho]] (kg/m3) u (mPa-s)
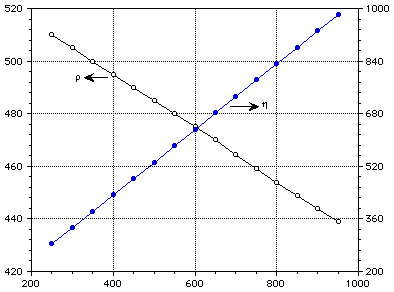
Temperature (C)
Figure 2 : Density and viscosity of pure liquid lithium.